- Received June 12, 2024
- Accepted September 26, 2024
- Publication February 15, 2025
- Visibility 14 Views
- Downloads 0 Downloads
- DOI 10.18231/j.ijogr.2025.004
-
CrossMark
- Citation
Introduction
Infertility is major problem in India. Infertility refers to the condition where a couple is not able to conceive after 12 months of unprotected sexual intercourse.[1] Approximately 5-15% of all married couples in country are facing this problem.[2] Generally, the social stigma is attached to female partner but around 30% males are also contributing to infertility.[2] Declining fertility rate is a serious concern in both males and females and needs urgent redressal.
Male factor infertility has been on rise recently and trend towards declining semen quality is revelation of deteriorating reproductive health.[3] It has promoted reproductive research to scrutinize the underlying causes to bring about effective therapeutic and preventive measures.
Several factors contribute to global declining trends in semen parameters however few pathological, environmental, and life style associated factors have been identified [4] which affect the hormonal milieu in the male reproductive organ thereby affecting spermatogenesis. Endogenous hormones play important role in regulation of male reproductive function by influencing steroid genesis or/and spermatogenesis process and in that purview role of thyroid hormones has been specifically investigated.[5]
According to studies Thyroid hormone leads to alteration in semen parameters by genomic and non-genomic pathways. Genomic effects are produced by binding of T3 to TR receptor in nucleus of Sertoli and Leydig cells. Non genomic effects result from binding of thyroid hormones to nonnuclear receptors present in the cytoplasmic membrane, cytoplasm, mitochondria and cytoskeleton of the sperm. This way it enhances the synthesis of cyclic adenosine monophosphate (cAMP) and increases release of calcium and ultimately affect sperm motility.[6] Thyroid hormones also contribute to regulation of the redox status of testis that relies on a number of antioxidant systems and is required for optimum spermatogenesis.[7]
The two most common types of thyroid dysfunction include hyperthyroidism and hypothyroidism; the former may result in oligozoospermia, asthenozoospermia, and/or teratozoospermia and reduced semen volume while later may lead to teratozoospermia and decreased sperm motility.[7] The affected parameters reverted to normal after the treatment.[8]
Several studies have been reported to evaluate the role of thyroid hormones on physiological male reproductive function but very few have targeted the direct effect of thyroid dysfunction on semen parameters and quality. Thus, in order to further evaluate the role of Thyroid hormones in optimizing spermatogenesis and to investigate the effect of Thyroid dysfunction on semen quality the study was undertaken.
Materials and Methods
We conducted a Prospective cohort study between November 2020 to November 2022. All the males of infertile couple attending infertility clinic outpatient department (OPD) of Jawaharlal Nehru Medical College and Hospital, Aligarh, Uttar Pradesh, India were taken as subjects. Ethical clearance from the Institutional Ethics Committee (IEC) of Jawaharlal Nehru Medical College and hospital was taken in order to conduct this study. Patients were enrolled after taking written informed consent and satisfying inclusion and exclusion criteria.
Inclusion criteria
All the males of infertile couple attending infertility clinic for couple infertility in reproductive age group
Exclusion criteria
Males having systemic diseases (such as diabetes, hypertension, cancer), Endocrinal abnormalities other than thyroid which may affect male reproductive function. Subjects diagnosed with any sexually transmitted diseases, Males undergoing therapies such as radiotherapy and chemotherapy, urogenital problems or any other serious chronic diseases that might have an impact on reproductive system were not included.
Calculation of sample size
The current study was carried by selecting the males attending the fertility clinic.
The sample size calculation was done by formula,
Sample size (n) = Z2PQ/d2
Z2 value is fixed at 95% of confidence interval was 1.96
Prevalence (P) = 7%, 0.0711 (based on previous studies)
Q = 1-P.
Error (d) is fixed at 5%., i.e., 0.05.
The sample size (n) = (1.96*1.96) *(0.07) *(0.93) / (0.05*0.05) = 0.2501 / 0.0025 = 100.04 => 100.
Therefore, the sample size = 100.
Total sample size with correction is 100.
SPSS version used for statistical analysis-26
Methodology
Men who attended infertility clinic were recruited after fulfilling the inclusion criteria. Detailed history was taken and physical examination performed. Baseline characteristics such as age, BMI (WHO classification 2014), alcohol addiction,[9] smoking Index,[10] educational qualification, and religious status were documented.
Semen samples and blood investigations were collected. Semen samples of male subjects were self-collected after 3-5 days of abstinence and following the prerequisites of WHO criteria 2021. Detailed evaluation of each sample was done in accordance with WHO recommendations 2021.
Blood samples were taken from patients using aseptic precautions and was evaluated for Hemogram. Serum TSH, and T4 levels were assessed using CLIA Kit (Chemiluminescence Immunoassay). TSH measurement is regarded as the most sensitive laboratory test for screening individuals for thyroid hormone abnormalities. This is due to the fact that small changes in free T4 levels result in larger changes in TSH values. American Thyroid Association was considered as the standard body of reference.
ATA reference range
Euthyroid: 0.4-4.0 milliunits per litre [m U/L]
Hyperthyroid :<0.4 milliunits per litre [m U/L]
Hypothyroid: >5.5 milliunits per litre [m U/L]
Results
In the present study the, multiple characteristics of the study population (N=100) were evaluated and tabulated in [Table 1].
|
Patient characteristics |
Frequency/Mean value |
Percentage |
|
Age |
34.27+5.3yrs |
|
|
BMI |
25.99+2.97 |
|
|
Alcohol |
8 |
8.00% |
|
Tobacco consumption |
32 |
32.00% |
|
Smoking |
22 |
22.00% |
|
Serum TSH(mU/L) |
||
|
Euthyroid |
73 |
73.00% |
|
Hyperthyroid |
3 |
3.00% |
|
Hypothyroid |
24 |
24.00% |
|
Mean ± SD |
3.36 ± 2.5 |
|
|
Median(25th-75th percentile) |
2.6(2.05-3.92) |
|
|
Range |
0-14.94 |
|
|
Total Sperm concentration (10⁶/mL) |
||
|
Abnormal |
33 |
33.00% |
|
Normal |
67 |
67.00% |
|
Mean ± SD |
34.67 ± 26.54 |
|
|
Median(25th-75th percentile) |
35(12.8-46.5) |
|
|
Range |
0-110 |
|
|
Total progressive Motility (%) |
||
|
Abnormal |
45 |
45.00% |
|
Normal |
55 |
55.00% |
|
Mean ± SD |
35.51 ± 24.65 |
|
|
Median(25th-75th percentile) |
30(15-58.5) |
|
|
Morphology (N) (%) |
40-60% |
In present study, mean age distribution of the study subjects (N=100) was 34.27+5.3 yrs. Mean BMI was 25.99+2.97. Majority of the population was literate (83%). Primary infertility was the major concern of the study population. The Mean value of serum TSH (mU/L) in study subjects was 3.39 ± 2.57 with median (25th-75th percentile) of 2.6(2.05-3.92).
Of 100 subjects, 22(22.00%) were smokers where 15 were moderate, 6 light and 1heavy smoker and 8(8.00%) were alcoholics where 7 were mild and 1 moderate drinker respectively. The number of Euthyroid cases were 73(73.00%), whereas 24(24.00%) and 3(3.00%) were hypothyroid and hyperthyroid respectively, diagnosed on the basis of serum TSH and T4 levels. No cases of subclinical hypothyroidism were reported.
Detailed semen analysis showed normal range of Total Sperm concentration (10⁶/mL) in 67.00% of cases while 33(33.00%) subjects had any one value below the reference range and thus were categorized as abnormal or Low. Mean value of Total sperm concentration (10⁶/mL) was 34.67 ± 26.54 while median (25th-75th percentile) was 35(12.8-46.5).
Percentage total motility in 55(55.00%) cases was normal, whereas 45(45.00%) of them had motility below the 5th percentile of reference ranges. Mean value of progressive motility (%) of study subjects was 35.51 ± 24.65 with median (25th-75th percentile) of 30(15-58.5).
The study population was further categorized in to Euthyroid (N=73), Hypothyroid (N=24) and Hyperthyroid (N=3) groups and various parameters were compared and analyzed in the three groups as given in [Table 2].
|
|
Euthyroid (N=73) |
Hypothyroid (N=24) |
Hyperthyroid (N=3) |
|
Mean Age (years) |
35±3.5 |
37.66±4.3 |
33.32±6 |
|
Mean BMI (kg/m 2 ) |
24.45±3.2 |
26.12±1.2 |
24.22±4.1 |
|
Mean TSH (IU) |
3.39+2.57 |
7.87±1.12 |
0.0383±2.4 |
|
Alcohol consumption |
3 (2 mild drinking, 1 moderate drinking) |
2 (2 mild drinking) |
3 (3 mild drinking) |
|
Smokers (Smoking Index >200 |
10 (6 moderate smokers, 3 light, 1 heavy smoker) |
6 (4 moderate, 2 light smokers) |
6 (3 moderate, 3 light smoker) |
|
Total sperm concentration (no/ml) |
39.6125±26.54 |
14.4029±18.14 |
17.6000±10.83 |
|
Total progressive Motility% |
40.751±24.64 |
14.4029±19.96 |
28±36.49 |
|
Semen volume (ml) |
2.71±1.46 |
2.87±2.1 |
2.5±1.5 |
|
Morphology (%) |
45-60% |
42-57% |
40-56% |
Mean age and BMI were comparable in all the groups using One-way ANNOVA test with p-value < 0.05. Qualitative variables namely smoking index and alcohol consumption were compared using Chi square test and no significant difference was noted (P-value 0.882 and chi-0.092).
Semen parameters were as follows, Mean value of Total sperm concentration (TSC) in Euthyroid, Hypothyroid and Hyperthyroid grp was 39.6125±26.54, 14.4029±18.14 and 17.6000±10.83 while Total percentage progressive motility was 40.751±24.64, 14.4029±19.96 and 28±36.49. Semen volume was 2.71+1.46, 2.87+2.1 and 2.5+1.5 respectively.
Correlation of TSH with semen parameters (TSC, sperm motility, semen volume and morphology (N=100)
The correlation between TSH and Total sperm cell concentration as well as motility in total study population (N=100) was established on application of Pearsons correlation and a significant strong negative correlation was found (r= -.276, p=0.005), while with motility, a significant moderately strong negative correlation (r=-.310, p=0.002) was found as demonstrated in [Table 3]. It can be postulated that higher values of TSH (Hypothyroidism) have inverse effect on Total sperm count and motility of sperms or vice versa. On the scatter plot diagram, the inverse correlation between thyroid levels and total sperm count and sperm motility can be seen. ([Figure 1], [Figure 2]).
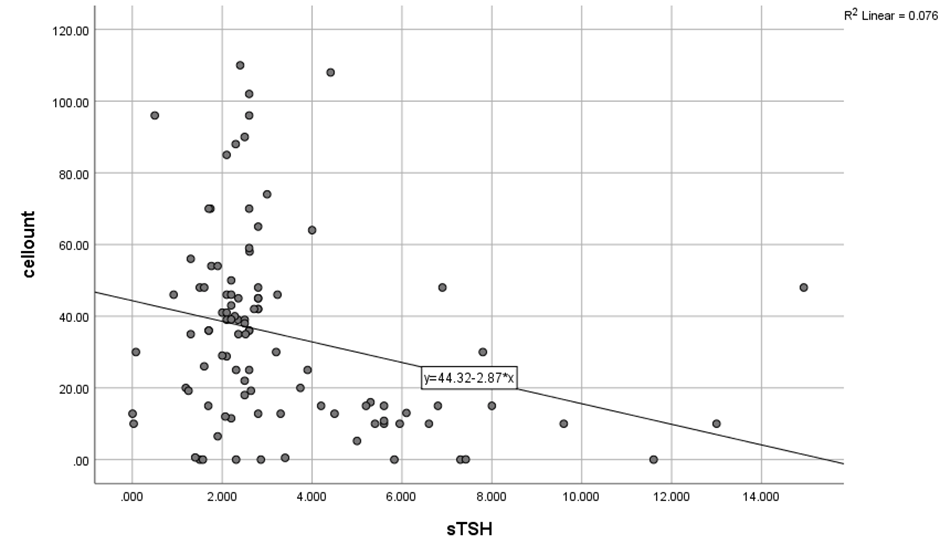
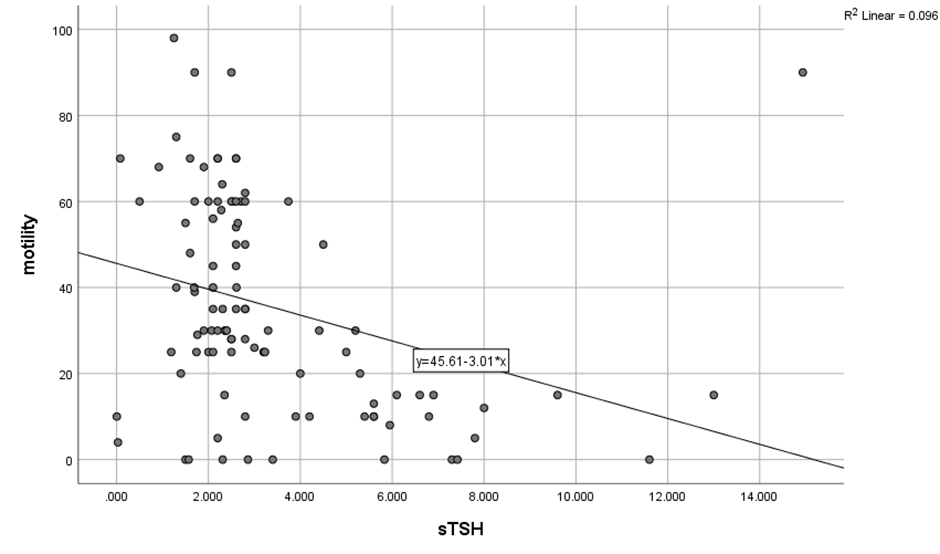
Mean serum TSH was 3.392±.57 and mean Semen volume was 2.71± 1.46 in (N=100) and on application of Pearson correlation test no correlation could be elucidated while similar results were obtained with morphology.
|
|
sTSH |
T.Sperm conc |
Motility |
|
|
sTSH |
Pearson Correlation |
1 |
-.276** |
-.310** |
|
Sig. (2-tailed) |
|
.005 |
.002 |
|
|
N |
100 |
100 |
100 |
|
|
Total sperm conc |
Pearson Correlation |
-.276** |
1 |
.482** |
|
P-value-Sig. (2-tailed) |
.005 |
|
.000 |
|
|
N |
100 |
100 |
100 |
|
|
Motility |
Pearson Correlation |
-.310** |
.482** |
1 |
|
P value-Sig. (2-tailed) |
.002 |
.000 |
|
|
|
N |
100 |
100 |
100 |
Effect of thyroid dysfunction (Hypothyroidism, hyperthyroidism) on semen parameters
As previously mentioned, the study population was divided in to Euthyroid, Hypothyroid and Hyperthyroid group with Mean TSC as 39.61, 14.4029 and 17.6 whereas Total sperm motility was 40.751+24.64, 14.05+19.96 and 28+36.49 respectively, mean Semen volumes noted in three groups were 2.81±12(E), 2.87+2.1(Hy), and 2.5.21(Hyr) and morphology was 45-60%, 42-57%, 40-56% in the respective groups, as shown in [Table 4].
|
Semen parameters |
Euthyroid N = 80 |
Hypothyroid N = 17 |
Hyperthyroid N = 3 |
p-value |
|
Motility |
40.751±24.64 |
14..05±19.96 |
28.3±36.49 |
>0.05 |
|
TSC |
39.6125 ±26.67 |
14.4029±14.66 |
17.60±10.82 |
>0.05 |
|
Volume |
2.81±12 |
2.87+2.1 |
2.5±21 |
<0.05 |
|
Morphology |
45-60% |
42-57% |
40-56% |
<0.05 |
Total sperm count/total sperm motility
Further Association was obtained using post-hoc analysis which demonstrated significant association between TSC and TSH in hypothyroidism group (p= 0.001, CI= (9.04-41.378) while it was not found to be significantly associated in hyperthyroidism. (p=.406, CI=-13.59-57.61). Similar results were found with respect to thyroid profile and sperm motility in hypothyroidism group where significant association (p= 0.001, CI= 11.95-41.49) was noted, whereas none was obtained in hyperthyroidism (p=1.00, CI= -20.09-44.94). Thus, it could be inferred that higher values of TSH as seen in Hypothyroidism is adversely affecting the sperm motility and Total sperm count. However, in Hyperthyroid group motility and TSC is affected negatively but not significantly. There was non-significant difference between means of aforementioned parameters in Hypothyroid and Hyperthyroid group as shown in [Table 5].
|
Bonferroni |
|||||||
|
Dependent Variable |
(I) Status of thyroid |
(J) status of thyroid |
Mean Difference (I-J) |
Std. Error |
Sig. |
95% Confidence Interval |
|
|
Lower Bound |
Upper Bound |
||||||
|
Total sperm count |
Euthyroid |
Hyporthyroidism |
25.20956* |
6.63701 |
.001 |
9.0404 |
41.3787 |
|
Hyperthyroidism |
22.01250 |
14.61468 |
.406 |
-13.5919 |
57.6169 |
||
|
Hypothyroidim |
Euthyroid |
-25.20956* |
6.63701 |
.001 |
-41.3787 |
-9.0404 |
|
|
Hyperthyroidism |
-3.19706 |
15.56273 |
1.000 |
-41.1111 |
34.7170 |
||
|
Hyperthyroidism |
Euthyroid |
-22.01250 |
14.61468 |
.406 |
-57.6169 |
13.5919 |
|
|
Hypothyroidism |
3.19706 |
15.56273 |
1.000 |
-34.7170 |
41.1111 |
||
|
Motility |
Euthyroid |
Hypothyroidism |
26.719* |
6.061 |
.000 |
11.95 |
41.49 |
|
Hyperthyroidism |
12.425 |
13.347 |
1.000 |
-20.09 |
44.94 |
||
|
Hypothyroiism |
Euthyroid |
-26.719* |
6.061 |
.000 |
-41.49 |
-11.95 |
|
|
Hyperthyroidism |
-14.294 |
14.213 |
.951 |
-48.92 |
20.33 |
||
|
Hyperthyroidism |
Euthyroid |
-12.425 |
13.347 |
1.000 |
-44.94 |
20.09 |
|
|
Hypothyroidism |
14.294 |
14.213 |
.951 |
-20.33 |
48.92 |
Mean Semen volumes noted in three groups were 2.81 ±12(E), 2.87+2.1(Hy), and 2.5(Hyr) respectively. No significant difference existed among the means of semen volume in all three groups however Pearson correlation applied separately between serum TSH and semen volume in three groups showed significant negative correlation in hypothyroid group (R-value -0.33 and p-value 0.009) while none was obtained in Euthyroid and Hyperthyroid groups (E-r-0.123; p - 0.222) (Hyp-r-0.58; p-value 0.87) showing thereby Hypothyroidism affects the semen volume negatively. Similarly, Percentage Morphology was not significantly different in three groups and no association was found between TSH and morphology in each group.
Discussion
In the study the prevalence of Male factor infertility as suggested by abnormal semen parameters was 33% however global data survey mentions it to be between 7-15%.[11] In 2019, the global prevalence of male infertility was estimated to be 56,530.4 thousand (95% UI: 31,861.5–90,211.7).[12] The higher values in our study may be attributed to the targeted population of infertile couple where expected fertility is diminished as compared to the general population.
The mean age of the study population was 34.27+5.3 yrs and it was in coherence with multiple studies where comparable age groups were documented[13], [14], [15] as this age group strives to obtain pregnancy and in the event of inability to conceive, reports promptly. In this study Age did not affect the semen quality, as suggested by Pearson correlation (Semen volume r-value 0.153 and p-value 0.129, TSC- r-value 0.058 and p-value 0.566, Motility r-value -0.04 and p-value 0.693) however other studies have reported statistically significant age-related declines in the aforementioned parameters, whereas others have reported no changes or rarely, even improvements in some parameters, with age.[16], [17], [18], [19], [20], [21]
This disparity may be due to heterogeneity of the data, racial and geographical differences.
Further mean BMI of the group was 25.99+2.97, belonged to overweight category and correlation with semen quality using Pearson Correlation showed negative but insignificant correlation with TSC and motility (TSC-r--0.104 and p- 0.305 motility r-value-0.111 and p-value 0.273). Several studies supported the theory of BMI (Obese grp) affecting semen quality[22], [23], [24] unlike ours. The difference was presumably due lack of subjects in obese grp.
Prevalence of Thyroid abnormalities namely Hypothyroidism and Hyperthyroidism in the study population was 24 and 3 respectively. Prevalence of aforementioned abnormalities among men in general population is around 1.9-6.2%.[25] This difference from the general data is due to specified infertile population being targeted where Thyroid dysfunction is presumably more. Moreover, Thyroid hormones are known to be responsible for optimum reproductive function and therefore the study was conducted to evaluate the effect of thyroid abnormalities on the semen quality.
As mentioned earlier study population was divided in to 3 groups based on Thyroid abnormalities (Euthyroid, Hypothyroid and Hyperthyroid)
Hypothyroidism
Our study showed significant negative correlation of TSH with semen volume, sperm count and motility in Hypothyroid group however morphology was unaffected. Several studies reported similar findings including Corrales Hernandez et al[26] who analyzed patients of primary hypothyroidism and concluded that semen quality is adversely affected by hypothyroidism with diminished motility and semen volume. Griboff et al[27] reported negative correlation of hypothyroidism with sperm motility only. J Kumar et al[28] showed improvement in semen parameters after treatment of hypothyroidism. SL Vignera and R Vita[29] reported decrease in semen volume and alteration in morphology.
Hypothyroidism may affect Sertoli cell maturation and cause Leydig cell dysfunction and thereby affect sperm production[30] which results in decreased total sperm number. It also leads to impairment of acrosome integrity and mitochondrial activity and thus diminished motility.[31]
Hyperthyroidism
Three subjects were diagnosed with hyperthyroidism where Semen abnormalities like decreased motility and lower TSC was present in 2 patients whereas 1 had decreased semen volume, However TSH and semen parameters showed no significant association in this group. Similar studies[32], [33], [34], [35] conducted have reported hyperthyroidism producing adverse effect on TSC and motility. Hyperthyroidism is also associated with impairment of sperm morphology, as well as a reduction of semen volume[36], [37] Although our study showed semen abnormalities in all three subjects but could not reach significance probably due to smaller number of Hyperthyroid patients.
Conclusion
Evaluation of the male partner is an essential element in assessment of infertile couple. The presence of subtle abnormalities in males may not manifest as abnormal semen analysis but may result in inability of the couple to conceive. Conversely semen abnormalities may not always result in infertility. Therefore, assessing the male partner is a tricky affair.
Hormonal assessment is not a part of recommendation unless specifically indicated. Evidences suggest that abnormal hormonal milieu may affect the semen quality, specifically thyroid abnormalities which may or may not manifest as grossly abnormal semen analysis and therefore refraining from assessing Thyroid status may delay the appropriate workup of the male partner.
Role of thyroid hormones in optimization of testicular function has been well elucidated in previous studies and is also highlighted in the present study.
To conclude male factors should be thoroughly evaluated with due significance to thyroid function assessment after proper counselling. The screening of thyroid function might be reconsidered in light of available evidences from the present study and should be further confirmed by large scale studies.
Source of Funding
None.
Conflict of Interest
None.
Acknowledgement
All the work and contributions are acknowledged.
References
- TJ Lindsay, KR Vitrikas. Evaluation and treatment of infertility. Am Fam Physician 2015. [Google Scholar]
- KC Lo, DJ Lamb, JF Strauss, RL Barbieri. The testis and male accessory organs. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management 2004. [Google Scholar]
- E Carlsen, A Giwercman, N Keiding, NE Skakkebaek. Evidence for decreasing quality of semen during past 50 years. BMJ 1992. [Google Scholar]
- P Sengupta. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol 2013. [Google Scholar]
- P Sengupta, S Dutta, E Krajewska-Kulak. The Disappearing Sperms: Analysis of Reports Published Between 1980 and 2015. Am J Mens Health 2017. [Google Scholar]
- GR Mendeluk, M Rosales. Thyroxin Is Useful to Improve Sperm Motility. Int J Fertil Steril 2016. [Google Scholar]
- SL Vignera, R Vita, RA Condorelli, LM Mongioì, S Presti, Se Benvenga. Impact of thyroid disease on testicular function. Endocrine 2017. [Google Scholar]
- GE Krassas, N Pontikides, V Deligianni, K Miras. A prospective controlled study of the impact of hyperthyroidism on reproductive function in males. J Clin Endocrinol Metab 2002. [Google Scholar]
- SP Baker, ER Braver, LH Chen, G Li, AF Williams. Drinking histories of fatally injured drivers. Inj Prev 2002. [Google Scholar]
- HFL Garretsen. Problem drinking: Prevalence determination, influencing factors and prevention options: Theoretical considerations and research in Rotterdam. 1983. [Google Scholar]
- CLR Barratt, L Björndahl, CJ DeJonge, DJ Lamb, FO Martini, R Mclachlan. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-Challenges and future research opportunities. Hum Reprod Update 2017. [Google Scholar]
- H Sun, TT Gong, YT Jiang, S Zhang, YH Zhao, QJ Wu. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY) 2017. [Google Scholar]
- AO Peter, AP Temi, AP Olufemi, OM Simidele, AS Adeniran. Pattern of Semen Parameters and Factors Associated with Infertility in Male Partners of Infertile Couples in Nigeria. Andrology (Los Angel) 2016. [Google Scholar]
- S Sangisapu, S Karunakaran, AK Pillai. Effectiveness of double wash swim-up versus double density gradient swim-up technique of sperm preparation in in vitro fertilization. J Evid Based Med Healthc 2017. [Google Scholar]
- Y Oguz, I Guler, A Erdem, MF Mutlu, S Gumuslu, M Oktem. The effect of swim-up and gradient sperm preparation techniques on deoxyribonucleic acid (DNA) fragmentation in subfertile patients. J Assist Reprod Genet 2018. [Google Scholar]
- MK Demirkol, O Barut, NT Dogan, MB Hamarat, S Resim. At What Age Threshold does the Decline in Semen Parameters Begin?. J Coll Physicians Surg Pak 2021. [Google Scholar]
- V Pino, A Sanz, N Valdés, J Crosby, A Mackenna. The effects of aging on semen parameters and sperm DNA fragmentation. JBRA Assist Reprod 2020. [Google Scholar]
- RA Condorelli, S Lavignera, F Barbagallo, A Alamo, LM Mongioi, R Cannarella. Bio-Functional Sperm Parameters: Does Age Matter?. Front Endocrinol (Lausanne) 2020. [Google Scholar]
- AW Tiegs, J Landis, N Garrido, RT Scott, JM Hotaling. Total Motile Sperm Count Trend Over Time: Evaluation of Semen Analyses From 119,972 Men From Subfertile Couples. Urology 2019. [Google Scholar]
- S Alshahrani, A Agarwal, M Assidi, AM Abuzenadah, D Durairajanayagam, A Ayaz. Infertile men older than 40 years are at higher risk of sperm DNA damage. Reprod Biol Endocrinol 2014. [Google Scholar]
- M Das, N Al-Hathal, M San-Gabriel, S Phillips, IJ Kadoch, F Bissonnette. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. J Assist Reprod Genet 2013. [Google Scholar]
- L Sekhavat, MR Moein. The effect of male body mass index on sperm parameters. Aging Male 2010. [Google Scholar]
- VS Waghmare, R Jiwane, SK Sadawarte, V Gajbhiye, AS Rahule. Effect of Increasing BMI on Routine Semen Parameters in Young Adult Males. J Cont Med A Dent 2014. [Google Scholar]
- AB Ajayi, BM Afolabi, DA Victor, I Oyetunji, A Atiba, J Ehichioya. Semen Parameters Associated With Male Infertility in a Sub-Saharan Black Population: The Effect of Age and Body Mass Index. J Gynecology Infertility 2018. [Google Scholar]
- VU Menon, KR Sundaram, AG Unnikrishnan, RV Jayakumar, V Nair, H Kumar. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc 2009. [Google Scholar]
- JJC Hernández, JMM García, LCG Diez. Primary hypothyroidism and human spermatogenesis. Arch Androl 1990. [Google Scholar]
- SI Griboff. Semen analysis in myxedema. Fertil Steril 1962. [Google Scholar]
- BJ Kumar, ML Khurana, AC Ammini, MG Karmarkar, MM Ahuja. Reproductive endocrine functions in men with primary hypothyroidism: Effect of thyroxine replacement. Horm Res 1990. [Google Scholar]
- La Vignera, S Vita, R Condorelli, R A. Impact of thyroid disease on testicular function. Endocrine 2017. [Google Scholar]
- N Patel, JA Kashanian. Thyroid dysfunction and male reproductive physiology. Semin Reprod Med 2016. [Google Scholar]
- RM Romano, SN Gomes, NCS Cardoso, NCS Cardoso, L Schiessl, MA Romano. New insights for male infertility revealed by alterations in spermatic function and differential testicular expression of thyroid-related genes. Endocrine 2017. [Google Scholar]
- HR Clyde, PC Walsh, RW English. Elevated plasma testosterone and gonadotropin levels in infertile males with hyperthyroidism. Fertil Steril 1976. [Google Scholar]
- GS Kidd, AR Glass, RA Vigersky. The hypothalamic-pituitarytesticular axis in thyrotoxicosis. J Clin Endocrinol Metab 1979. [Google Scholar]
- M Abalovich, O Levalle, R Hermes, H Scaglia, C Aranda, C Zylbersztein. Hypothalamic-pituitary-testicular axis and seminal parameters in hyperthyroid males. Thyroid 1999. [Google Scholar]
- GE Krassas, N Pontikides, V Deligianni, K Miras. A prospective controlled study of the impact of hyperthyroidism on reproductive function in males. J Clin Endocrinol Metab 2002. [Google Scholar]
- SL Vignera, R Vita. Thyroid dysfunction and semen quality. Int J Immunopathol Pharmacol 2018. [Google Scholar]
- K Vaghelaa, H Oza, V Mishra, A Gautam, Y Verma, S Mishra. Relationship between Thyroid Profile with Reproductive Hormones and Semen Quality. Am Sci Res J Eng Technol Sci 2016. [Google Scholar]
- Introduction
- Materials and Methods
- Results
- Correlation of TSH with semen parameters (TSC, sperm motility, semen volume and morphology (N=100)
- Effect of thyroid dysfunction (Hypothyroidism, hyperthyroidism) on semen parameters
- Total sperm count/total sperm motility
- Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
- Acknowledgement
